Chronic Graft vs. Host Disease (cGvHD) remains a major debilitating and potentially fatal sequelae of allogeneic hematopoietic cell transplantation. Corticosteroids remain the initial therapy of clinically significant cGvHD. Since 2017 three targeted therapies have been approved for steroid refractory cGVHD, ruxolitinib, a JAK 1/2 inhibitor, is approved post one or two previous therapies. Ibrutinib, a Bruton's tyrosine kinase (BTK) inhibitor, and belumosudil, a ROCK2 inhibitor, are both approved after two previous lines of therapy.
We tested RR- RR-1752 a single digit nM dual ROCK/AURK inhibitor in a mouse model of severe cGVHD. BALB/c recipient mice were preconditioned with 7.5 Gy TBI, 8 hours later they received 10 7 T-cell depleted (TCD) C57BL/6 bone marrow cells alone (Group A, n=7) or 10 7 TCD C57Bl bone marrow cells plus 10 6 splenocytes (Groups B, n=8 and C, n=8). Beginning day 7 post cell infusion animals received either oral vehicle control (groups A and B) or vehicle with 1.0 mg/kg RR-1752 (Group C) daily. Body weight, alopecia score and eye score were monitored 3×/week through day 56 or until termination.
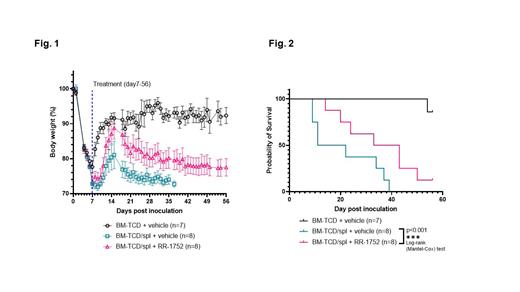
All animals in groups B and C developed signs and symptoms of chronic graft vs. host disease. Animals in group A exhibited 20% weight loss through seven days post irradiation and cell infusion but began to recover to baseline and recovered weight to 90% of baseline. Animal weights in groups B and C dropped to less than 80% at day seven post irradiation, but only recovered to 80.38% +/- 10.18 (Group B) and 87.11% +/- 7.90 (Group C) on day 14 and then declined to 74.10% +/- 4.19 in Group B and 80.23% +/- 7.35 in Group C by day 28 (Figure 1). Animals in Group A had no significant change in eye score or alopecia. In contrast all animals in groups B and C showed signs of cGvHD. Eye showed moderate effects in Group B and mild changes in group C, while alopecia scores were moderate in both groups B and C. All animals in group A survived through day 56, median survival in group B was 16.5 days, as compared to 38 days in group C (Figure 2).
In a separate study in normal mice, RR-1752 given at 1.0 mg/kg/day for 2 weeks showed no negative effects on hemoglobin, hematocrit, platelets, total WBC, or neutrophils, and only a mild decrease in lymphocytes.
Using a model for cGVHD, exhibiting weight loss, alopecia, and ocular changes, we demonstrated that RR-1752 1.0 mg/kg/day slows mouse body weight loss, increases survival and shows trends to ameliorate ocular toxicity. The dose tested did not ameliorate the alopecia in the cGVHD mice. Further studies are ongoing to assess higher dose levels and dose schedule effects in the severe cGVHD mouse model. Additional studies to compare effects on cGVHD to ruxolitinib and belumosudil and in combination with those standard of care therapies need to be conducted.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal